BACKGROUND
For patients with triple-class exposed/refractory multiple myeloma (TCE/R MM), prognosis is poor and limited effective treatment options are available. Elranatamab is a novel B-cell maturation antigen (BCMA)- and CD3-directed bispecific antibody that demonstrated safety and efficacy in patients with TCE/R MM in the phase 2, single-arm MagnetisMM-3 trial (NCT04649359). To compare the effectiveness of elranatamab vs physician's choice of treatment (PCT) in the absence of comparative data, an unanchored matching-adjusted indirect comparison (MAIC) was conducted.
METHODS
Individual patient data (IPD) from the 14.7-month follow-up of MagnetisMM-3 (Cohort A [BCMA-naïve] N=123) was matched to published summary data from two real-world studies of PCT: LocoMMotion ([NCT04035226]), an ongoing, prospective, non-interventional study of PCT in patients with TCE MM (N=248); and MAMMOTH, a multicenter, retrospective study investigating the history and outcomes of patients with TCE MM who received subsequent therapies (N=177). To adjust for differences in baseline characteristics, patients from MagnetisMM-3 were reweighted to match those in LocoMMotion reported in Mateos et al (2022) and MAMMOTH reported in Bal et al (2022). The base-case adjustment variables were selected based on univariate Cox regressions using the MagnetisMM-3 IPD, a systematic literature review of prognostic variables and effect modifiers in RRMM, a review of the prognostic variables identified in clinical studies for TCE/R MM, a review of the recent analogous indirect comparisons, and confirmation by clinical experts.
Weights were determined using a propensity score-type logistic regression via the method of moments (Signorovitch et al. 2012) based on age, median time since diagnosis, International Staging System disease stage, high-risk cytogenetics, extramedullary disease, number of prior lines of therapy, Eastern Cooperative Oncology Group performance status, creatinine clearance, and penta-exposed and penta-refractory status (where available). In the analysis for the endpoint of overall survival (OS), sex was also included in the analysis. A sensitivity analysis was conducted in which missing values of the adjusted baseline characteristics for elranatamab were imputed by a random sample of the observations in MagnetisMM-3 to potentially increase the effective sample size (ESS).
Unanchored MAIC analyses used the R Studio statistical code provided in the National Institute for Health and Care Excellence Decision Support Unit 18 by Phillippo et al (2016) . In the MAIC vs LocoMMotion & MAMMOTH, adjusted objective response rate (ORR), complete response rate or better (≥CR), progression-free survival (PFS), and OS at 15 months for elranatamab were compared with PCT. Results were reported as rate differences, which is the difference between the ORR or ≥CR rates of elranatamab and PCT, and odds ratios (ORs) for binary endpoints and hazard ratios (HRs) for time-to-event endpoints, with two-sided 95% confidence intervals (CIs).
RESULTS
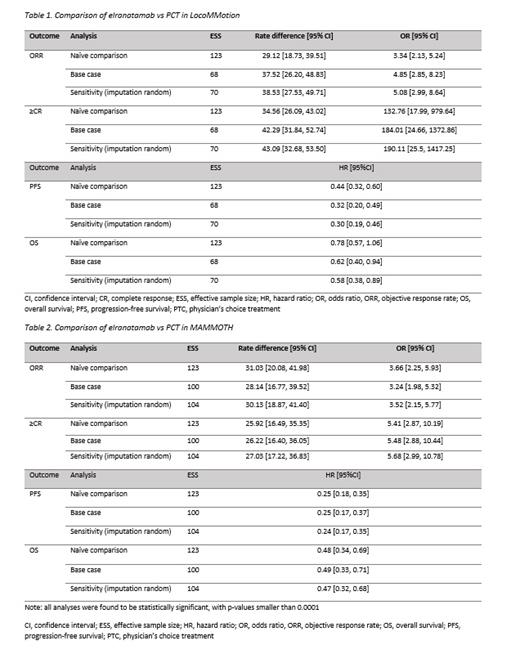
In the MAIC for elranatamab vs PCT in LocoMMotion, the post-matching ESS was 68 for all endpoints in the base case. Compared with PCT in LocoMMotion, elranatamab was associated with a significantly higher ORR (rate difference: 37.52; 95% CI 26.20-48.83; OR: 4.85; 95% CI 2.85-8.23), higher ≥CR (rate difference: 42.29; 95% CI 31.84-52.74; OR: 184.01; 95% CI 24.66-1372.86), and longer PFS (HR 0.32; 95% CI 0.20-0.49) and OS (HR 0.62; 95% CI 0.40-0.94) (Table 1). In the MAIC vs PCT in MAMMOTH, the base-case post-matching ESSs were 100 for all endpoints. Compared with PCT in MAMMOTH, elranatamab was associated with a significantly higher ORR (rate difference: 28.14; 95% CI 16.77-39.52; OR: 3.24; 95% CI 1.98-5.32) and ≥CR (rate difference: 26.22; 95% CI 16.40-36.05; OR: 5.48; 95% CI 2.88-10.44). Elranatamab also had longer PFS (HR 0.25; 95% CI 0.17-0.37) and OS (HR 0.49; 95% CI 0.33-0.71) than PCT (Table 2). Sensitivity analysis results were consistent with the base case.
CONCLUSIONS
In this MAIC, elranatamab demonstrated significantly higher ORR, higher ≥CR rate, longer PFS, and longer OS compared with PCT in the real-world. These results suggest that elranatamab offers significantly improved outcomes versus standard treatments used in patients with TCE/R MM in clinical practice.
Disclosures
Mol:Pfizer Inc: Consultancy. Hu:Pfizer Inc: Consultancy. LeBlanc:Astellas: Consultancy, Honoraria, Speakers Bureau; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Speakers Bureau; Agilix: Consultancy, Honoraria; UpToDate: Patents & Royalties; American Cancer Society: Research Funding; Incyte: Honoraria, Speakers Bureau; Dosentrx: Current equity holder in private company; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Meter Health: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; Flatiron: Consultancy, Honoraria; CareVive: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; BlueNote: Consultancy, Honoraria; Deverra Therapeutics: Research Funding; Duke University: Research Funding; Jazz Pharmaceuticals: Research Funding; Leukemia and Lymphoma Society: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; National Institute of Nursing Research/National Institutes of Health: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria. Cappelleri:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Chu:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Nador:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Aydin:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Schepart:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Hlavacek:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal